
The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

PDF) Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance | Ataah T - Academia.edu

POL-0030: Compliance and enforcement approach and inspection strategy for clinical trials of drugs involving human subjects - Canada.ca

All About Clinical Research : Word Search and Flash Cards for Ich Guidelines for Good Clinical Practice: (Travel Size 2Nd Edition) a Study Guide for the International Council for Harmonisation of Technical
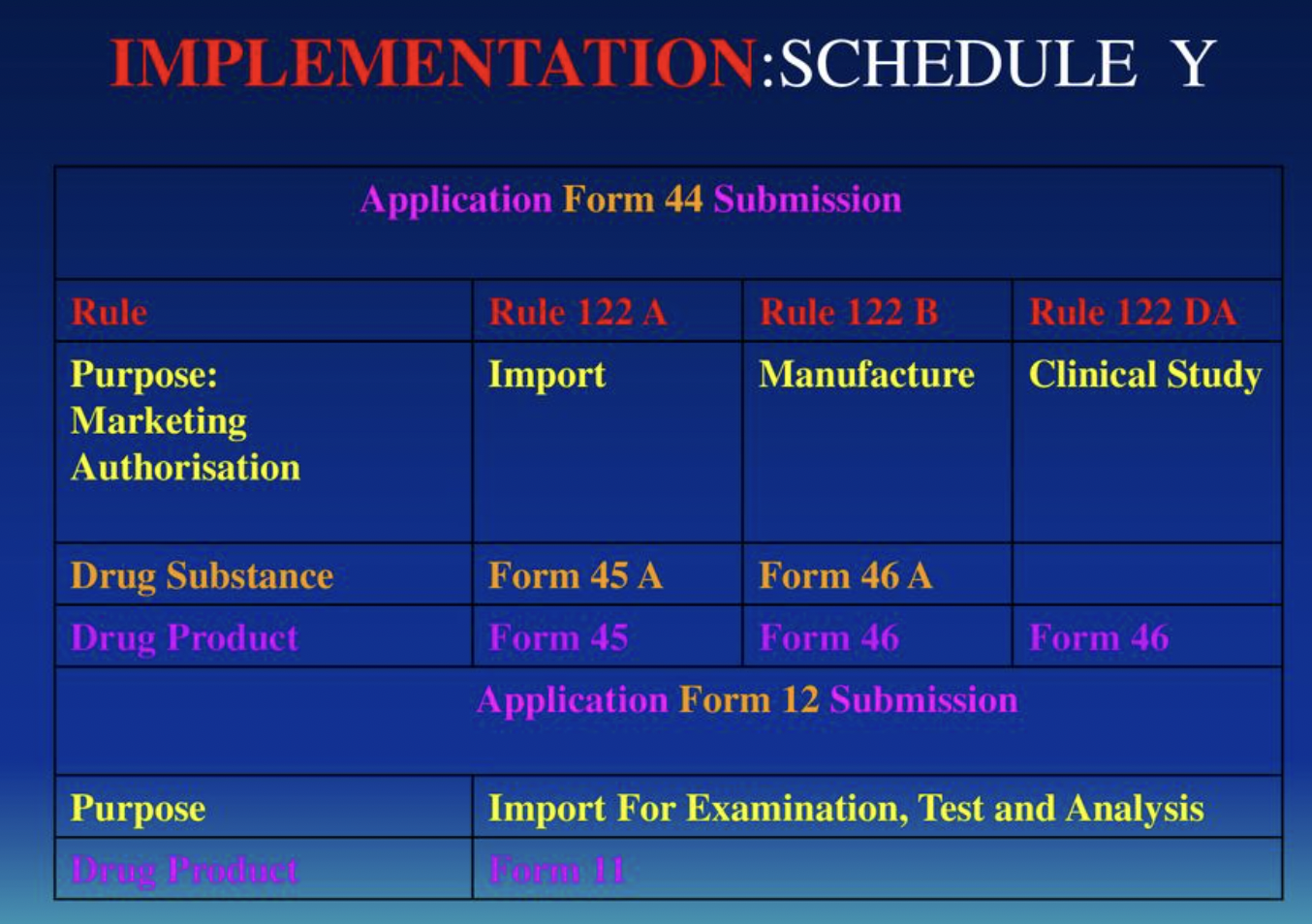
difference between ich gcp indian gcp and schedule y ppt — Clinical Research Certification I Blog - CCRPS
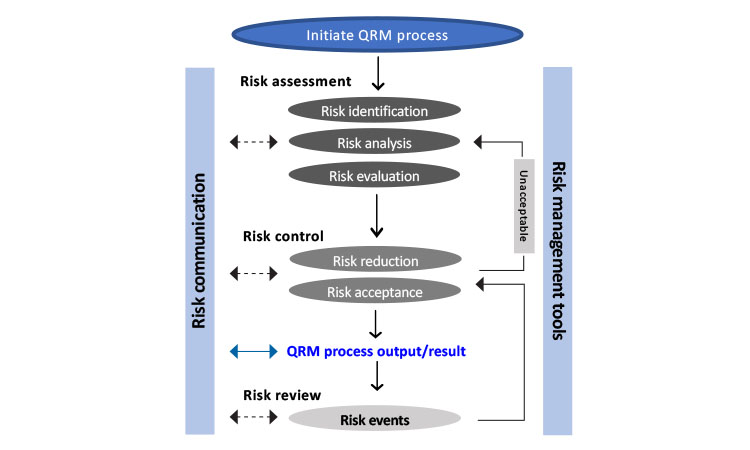
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering

US FDA Guidance – Good Clinical Practice: Integrated Addendum to ICH E6(R1) - Lachman Consultant Services, Inc.

in the united states following the ich e6 guideline is — Clinical Research Certification I Blog - CCRPS













